Bournemouth University


![]()
Biomedical engineering research at Bournemouth University, in conjunction with Salisbury NHS Foundation Trust, over many years has developed intelligent Functional Electrical Stimulation (FES) systems – a method of externally controlling muscles – which are improving the quality of life for people with neurological disabilities across the globe.
Signals from the brain are crucial to control how we move our bodies. But countless lives each year are devastated when those signals cannot get through because of a spinal cord injury or a neurological disease such as Multiple Sclerosis, or due to damage to the brain following a stroke.
The research team’s FES devices, developed through a long-term relationship with Odstock Medical Ltd (OML) – the first NHS commercial company in England which was established in 2006 – have significant advances over traditional physiotherapy alone. Bournemouth University originally provided a rapid prototyping facility via the Product Design Service and, through a succession of PhD students’ research on body area wireless networks and other engineering techniques, have sought to maximise the clinical and therapeutic benefits of the system.
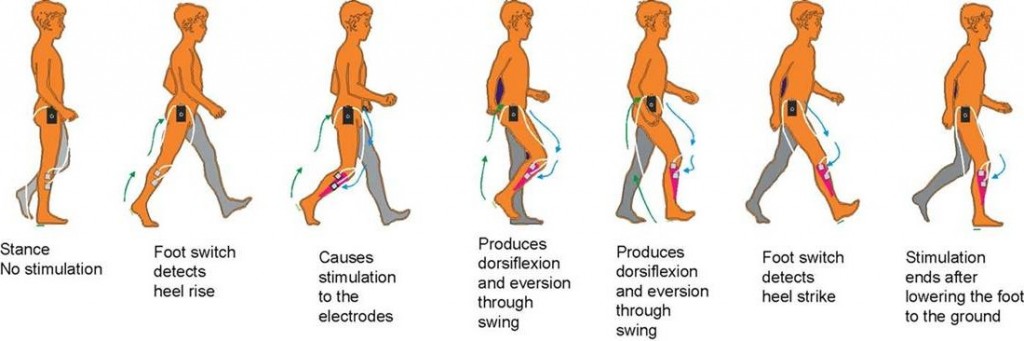
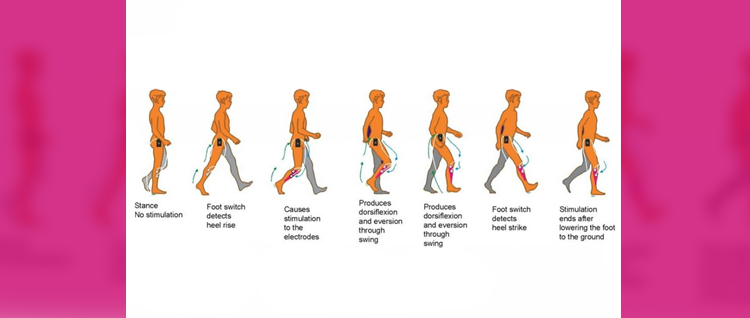
FES can radically improve people’s quality of life by increasing functional ability, reducing isolation and dependence on others. One of the most common applications of the device is for the correction of ‘dropped foot’ in people with MS or following a stroke. This is where a patient’s toes catch on the ground as they walk along, making it more difficult to move and hence more likely to trip or fall. FES is able to overcome foot drop by the use of small electrical signals to replace the nerve impulses that have been interrupted by damage to the brain and/or spinal cord.
This use of FES for dropped foot obtained National Institute for Health and Care Excellence (NICE) approval in 2009 and the Royal College of Physicians have positively commented on the devices in their 2012 Stroke Guidelines.
Since 2008, well over 10,000 stimulators have been sold – doubling OML’s turnover. They have also been exported to 18 countries including developing nations such as India. The company aims to make FES a routine treatment in the NHS and to develop its use overseas. They are already working with the research team to improve the current technology through further study and new techniques. Their vision is to produce a new range of FES devices, underpinned by BU’s research expertise, which will lead the world in providing innovative solutions and further improved quality of life for people with a wide range of neurological disabilities and conditions.