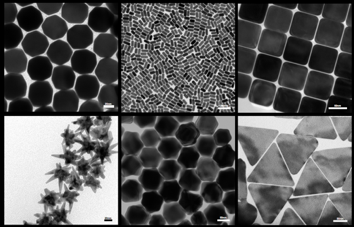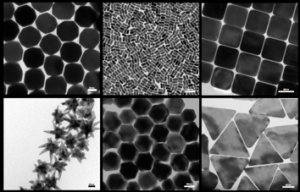
Transmission Electron Microscopy micrographs of the library of gold nanoparticles, developed by Dr Zeljka Krpetic
The survival rate of UK cancer patients has doubled in the last half century. But non-selective radiation treatment may cause damage to healthy cells and tissue.
In Salford NanoLab, within the School of Environment and Life Sciences at the University of Salford, Dr Krpetic’s research team is working to minimise this through nanotechnology.
The use of nanotechnology in cancer treatment and diagnostics is not new.

Dr Zeljka Krpetic, lecturer in Physical Chemistry, University of Salford
To date, several kinds of nanoparticle carriers (nanomedicine) have been developed with the aim of providing targeted drug delivery. Each of them is designed to work in realistic biological environments and be effective when released in relevant biological fluids.
Understanding how nanomaterials interact with our bodies is key to their safe implementation in future medical treatments.
Why are we using gold? Gold has special properties, which have attracted attention for many centuries.
Gold nanoparticles – just like gold bars and jewellery – have extremely low or negligible toxicity to human cells. And due to its high stability, gold nanoparticles can be easily functionalised with cancer targeting molecules using a variety of approaches.
This biocompatibility along with the ability to specifically locate them at tumor sites make them an ideal therapeutic contrast agent.
Gold nanoparticles have previously been approved as successful X-ray contrast agents and have an excellent track record in tumour-targeting, achieved through multiple bioconjugation techniques which allow them to carry a variety of drugs or tags capable of tackling the cancer.
The preferential delivery of functionalised GNPs into tumours, as shown in previous studies with mice, leads to an improvement in tumour control and treatment following radiotherapy.
At Salford, we are seeking to identify “gold nanobullets” that will improve current radiation treatments by minimising the radiation dose.
Find out more about the Doctoral Training Alliance (DTA).